WHO Resumes Coronavirus Trial On Malaria Drug Hydroxychloroquine After Examining Safety
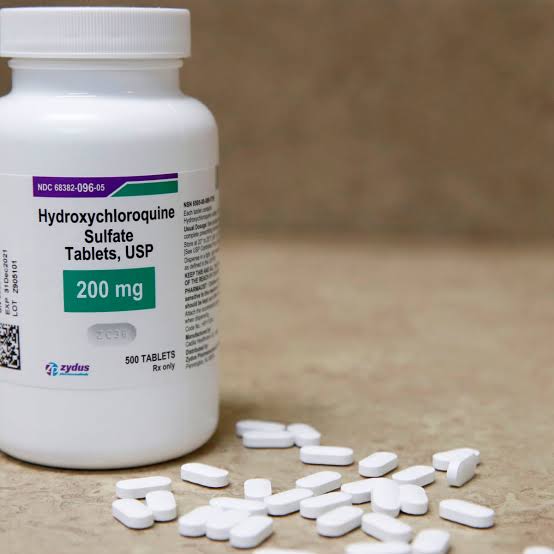
World Health Organization has resumed its trial of hydroxychloroquine, the malaria drug dubbed as a potential treatment for coronavirus, after temporarily halting research over safety concerns.
WHO Director-General Tedros Adhanom Ghebreyesus made the announcement during a press conference on Wednesday at the agency’s Geneva headquarters, Switzerland.
The Data Safety Monitoring Board, according to Ghebreyesus, decided there is no reason to discontinue the international trial after reviewing available data on the drug.
WHO DG said;
The executive group received this recommendation and endorsed the continuation of all arms of solidarity trial including hydroxychloroquine.
On May 25, WHO announced it had temporarily suspended its trial of the drug over safety concerns.
Read Also: COVID-19: WHO Suspends Trial Of Hydroxychloroquine Over Safety Concerns
The decision to suspend the trial came after a study published in medical journal The Lancet found that hospitalized COVID-19 patients treated with hydroxychloroquine had a higher risk of death than those who did not take it.
In light of the recent development, the study is now being reexamined.
Speaking further on Wednesday, WHO officials said there is no evidence that any drug actually reduces the mortality in patients who have COVID-19.
Tedros stated the safety board will continue to closely monitor the safety of all therapeutics being tested in its trials, which involve more than 3,500 patients across 35 countries.
He expressed;
WHO is committed to accelerating the development of effective therapeutics, vaccines and diagnostics as part of our commitment to serving the world with science, solutions and solidarity.
Apart from being an anti-malaria drug, hydroxychloroquine is often used by doctors to treat rheumatoid arthritis and lupus.
It is known to have serious side effects, including muscle weakness and heart arrhythmia. Numerous clinical trials are looking to see if it is effective in fighting COVID-19, but it is not a proven treatment.
A week ago, WHO said there was no evidence that the drug is actually effective against the coronavirus.
Dr. Mike Ryan, executive director of the WHO’s emergencies program on May 27 expressed that;
We do not advise the use of hydroxychloroquine or chloroquine for the treatment of Covid-19 outside randomized control trials or under appropriate close clinical supervision subject to whatever national regulatory authorities have decided.
Therefore, hydroxychloroquine is only approved for use during clinical trials and not for self-medication.
